2006 The former Hitachi Cable News Release
Information (including product prices, product specifications, details of services, launch dates, inquiry information, and URLs) contained in this news release is current as of the date of the press release but is subject to change without notice. Please note that details may differ from those effective on the search date.
Development of Continuous Processing Technology for Low-Cost Recycling of Cross-linked Polyethylene Using Supercritical Alcohol
-Research commissioned by NEDO-
As a fundamental technology development project commissioned by NEDO (New Energy and Industrial Technology Development Organization), Hitachi Cable, Ltd. recently developed a continuous processing technology that enables low-cost recycling of silane cross-linked polyethylene. Hitachi Cable has sought to develop and industrialize a production technology that would allow low-cost recycling of silane cross-linked polyethylene waste generated during the manufacture and disposal of power cables for use as insulating materials for the production of power cables.
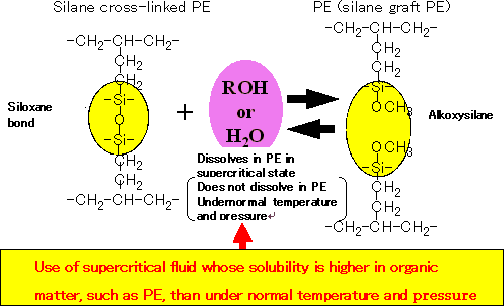
Some 10,000 tons of silane cross-linked polyethylene waste is expected to be generated annually in Japan from the disposal of power cables and other materials. Currently, most silane cross-linked polyethylene waste is converted into fuel or buried as landfill. But mounting environmental concerns mandate the effective use of natural resources, including the recycling and reuse as a raw material of power cable sheaths. In collaboration with Hitachi, Ltd., National Institute of Advanced Industrial Science and Technology (AIST), and The Japan Steel Works, Ltd., Hitachi Cable in 2004 discovered a way to transform non-thermoplastic silane cross-linked polyethylene waste into meltable, formable thermoplastic polyethylene involving the application of a supercritical alcohol (*1 , Fig. 1) to silane cross-linked polyethylene (See Fig. 2).
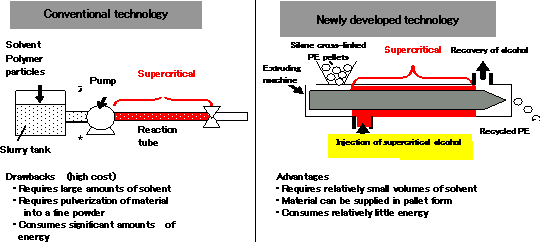
Conventionally, polymer processing using supercritical alcohol involves a combination of a high-pressure pump and a reaction tube, a method that requires pulverization of raw polymer into fine powder. In addition, this method requires a large amount of solvent heated to high temperatures, making processing of polymer quite costly (See Fig. 3). A continuous processing technology was needed that would enable low-cost production and permit industrialization of the method.
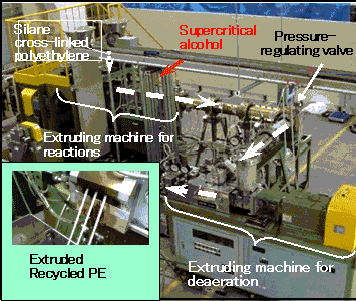
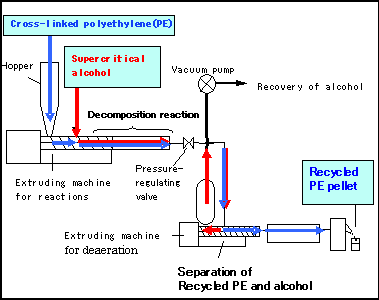
Through active research into the fundamental technologies for low-cost production, Hitachi Cable has developed a technology that permits a continuous recycling process of cross-linked polyethylene using an extruding machine commonly used to mix and form resins in a supercritical fluid reaction vessel, injecting a supercritical alcohol into the extruding machine during the process (See Fig. 4). This method does not require polymer pulverization and permits the direct feeding of polymer pellets *2 into the extruding machine. It also uses relatively small amounts of solvent, significantly reducing energy consumption.
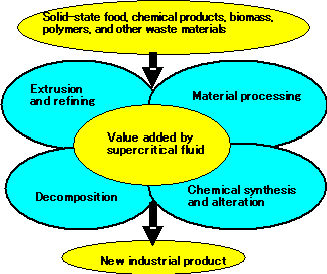
This recently developed continuous processing technology incorporating a supercritical fluid is expected to find applications not just in material recycling, but in a broad range of industrial areas, including biomass technologies and the development of new materials based on polymer alteration (See Fig. 5). The breakthrough by Hitachi Cable was made possible by the cooperation of National Institute of Advanced Industrial Science and Technology (AIST) and Shizuoka University. In collaboration with these research organizations, Hitachi Cable plans to continue research to ensure the practical application and safety of the test equipment.
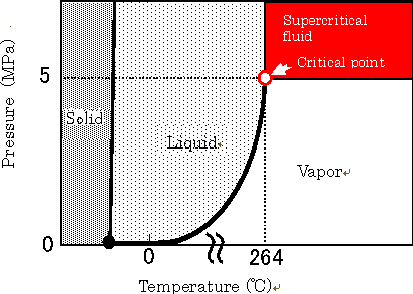
| *1 | Supercritical alcohol: A supercritical state refers to a condition in which the limit temperature and pressure (critical point) allowing the coexistence of vapor and liquid states is exceeded, rendering the density of the vapor and liquid equivalent. In this state, materials in two different phases can no longer be distinguished. Because supercritical alcohol has both vapor diffusivity and liquid solubility, it provides a variety of actions as a reaction solvent. |
| *2 | Pellet: A plastic sphere or grain of about 5 mm in size. |
Fig.1 Supercritical fluid (state diagram of alcohol)
Fig.2 Chemical reaction of siloxane bond
Fig.3 Comparison of continuous processing method
Fig.4 System for continuous processing of silane cross-linked polyethylene using supercritical alcohol
(a)External view of the system
(b) System diagram
Fig.5 Projected applications of supercritical fluid continuous process using extruding machines